FDA Clears Three Dräger Ventilators
Von einem Mystery-Man-Autor
Last updated 23 mai 2024

Dräger has received 510(k) clearance from the US FDA for its Evita V600, V800, and Babylog VN800, ventilators.

Draeger Breathing Filter Gets Class I Recall
DRAGER V500 designed for Adult - Pediatric and infant use. The sale of this item may be subject to regulation by the U.S. Food and Drug Administration

DRAGER V500 Adult - Pediatric VENTILATOR
((InvIDs))279722(((InvIDs))). Is a lift gate truck needed?. The sale of this item may be subject to regulation by the U.S. Food and Drug

DRAGER EVITA 4 VENTILATOR % (279722)

Medical Device News Update - August 2023 - Let's Talk Risk!

Drager Evita XL Ventilator - For Sale — Integris Equipment LLC

Elevating Firefighting Safety Through Ergonomic Innovation: Dräger Introduces the HPS SafeGuard Helmet

Draeger's ventilators with contaminated breathing gas in Class I recall - Medical Device Network

FDA Recall: VentStar and ID Breathing Circuits, Anesthesia Sets - Pulmonology Advisor
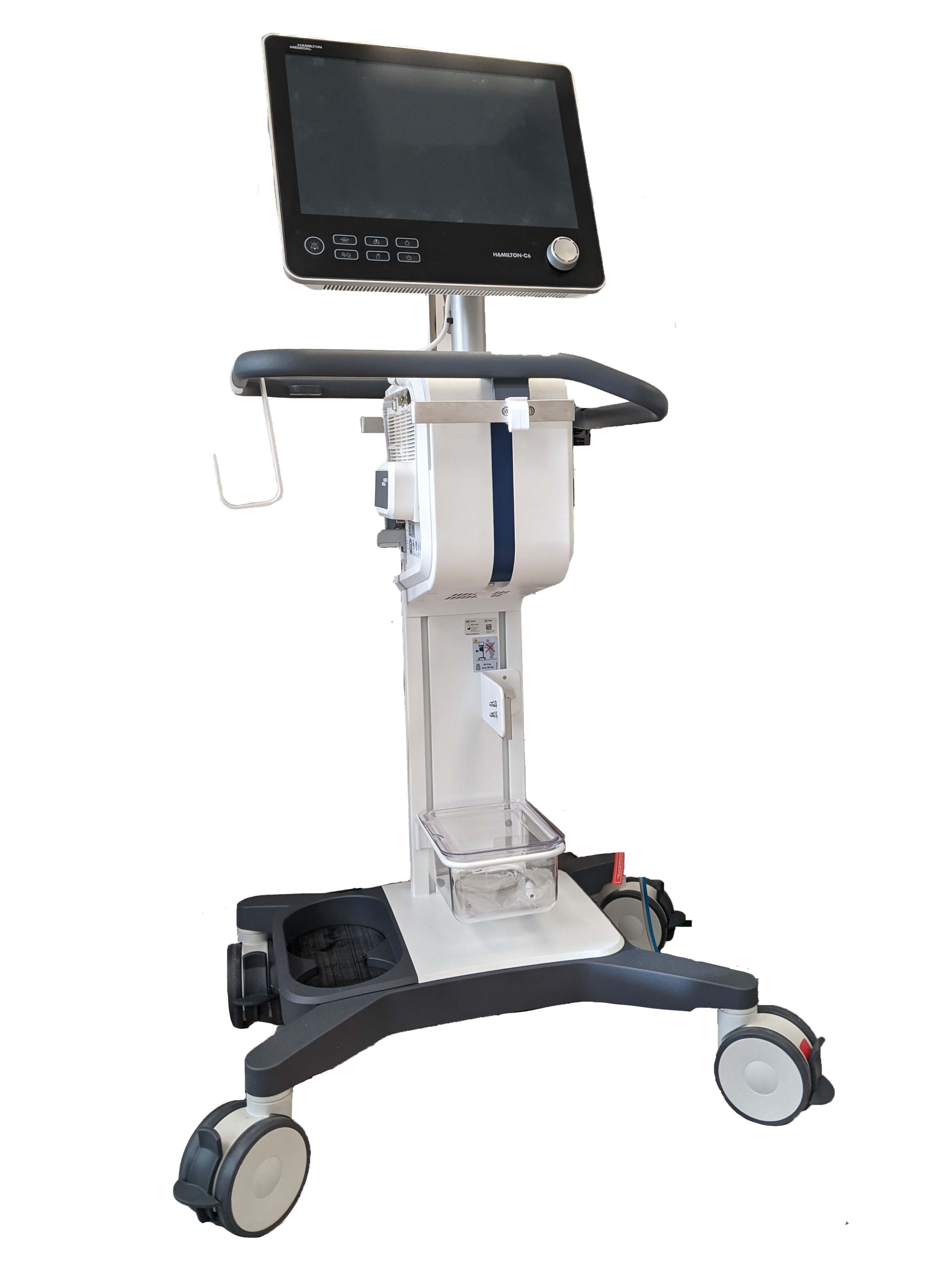
Ventilator - Wikipedia

Coronavirus (COVID-19 or nCoV) - Dräger's Important Information during the Novel Coronavirus Outbreak

Draeger recalls Seattle-PAP Plus and breathing circuit kits
für dich empfohlen
 Dräger Alcotest® 382014 Jul 2023
Dräger Alcotest® 382014 Jul 2023 Dräger Global14 Jul 2023
Dräger Global14 Jul 2023 Alcootest 4000® Dräger Ethylotest électronique14 Jul 2023
Alcootest 4000® Dräger Ethylotest électronique14 Jul 2023- Dräger Anesthesia - iFixit14 Jul 2023
 Dräger Interlock® 700014 Jul 2023
Dräger Interlock® 700014 Jul 2023 Videos Dräger14 Jul 2023
Videos Dräger14 Jul 2023 Dräger – ServiceNow – Erfolgsgeschichte14 Jul 2023
Dräger – ServiceNow – Erfolgsgeschichte14 Jul 2023 Dräger Feuerwehrhelm HPS 3500 nachleuchtend, Feuerwehr, Ausrüstung, Unsere Produkte14 Jul 2023
Dräger Feuerwehrhelm HPS 3500 nachleuchtend, Feuerwehr, Ausrüstung, Unsere Produkte14 Jul 2023 Dräger Safety Bag14 Jul 2023
Dräger Safety Bag14 Jul 2023 Mohamed Dräger stellt sich vor FC Basel - Die offizielle Website14 Jul 2023
Mohamed Dräger stellt sich vor FC Basel - Die offizielle Website14 Jul 2023
Sie können auch mögen
 Für BMW E90 E91 2008-2011 E92 E93 2010-2013 Schwarz glänzende Rückspiegelabdeckung Ersatz links und14 Jul 2023
Für BMW E90 E91 2008-2011 E92 E93 2010-2013 Schwarz glänzende Rückspiegelabdeckung Ersatz links und14 Jul 2023 HELLA 1GA 996192001 LED Arbeitsscheinwerfer Power Beam 3000 12/2414 Jul 2023
HELLA 1GA 996192001 LED Arbeitsscheinwerfer Power Beam 3000 12/2414 Jul 2023 Bong Dab Rig 8 Design Perc14 Jul 2023
Bong Dab Rig 8 Design Perc14 Jul 2023 The Importance of Spending your Summer Wisely - Wealthy Habits14 Jul 2023
The Importance of Spending your Summer Wisely - Wealthy Habits14 Jul 2023 Creasono Produkte MP3-AUTORADIO (1-DIN) MIT BLUETOOTH UND VIDEO-ANSCHLUSS14 Jul 2023
Creasono Produkte MP3-AUTORADIO (1-DIN) MIT BLUETOOTH UND VIDEO-ANSCHLUSS14 Jul 2023 Amtra Netzteil 24V DC LED System - Olibetta14 Jul 2023
Amtra Netzteil 24V DC LED System - Olibetta14 Jul 2023 Autositzbezug Schonbezug, Vordersitzbezüge, VW Caddy, Grau14 Jul 2023
Autositzbezug Schonbezug, Vordersitzbezüge, VW Caddy, Grau14 Jul 2023- FORD LED LOGO PROJEKTOR FORD S-MAX MONDEO MK4 HD za 303 Kč - Allegro14 Jul 2023
 Car Wash Brush Long Handled14 Jul 2023
Car Wash Brush Long Handled14 Jul 2023 Pare-choc avant Ford Focus phase 2 tuning14 Jul 2023
Pare-choc avant Ford Focus phase 2 tuning14 Jul 2023
