Membrane binding of pore-forming γ-hemolysin components studied at different lipid compositions - ScienceDirect
Von einem Mystery-Man-Autor
Last updated 16 mai 2024

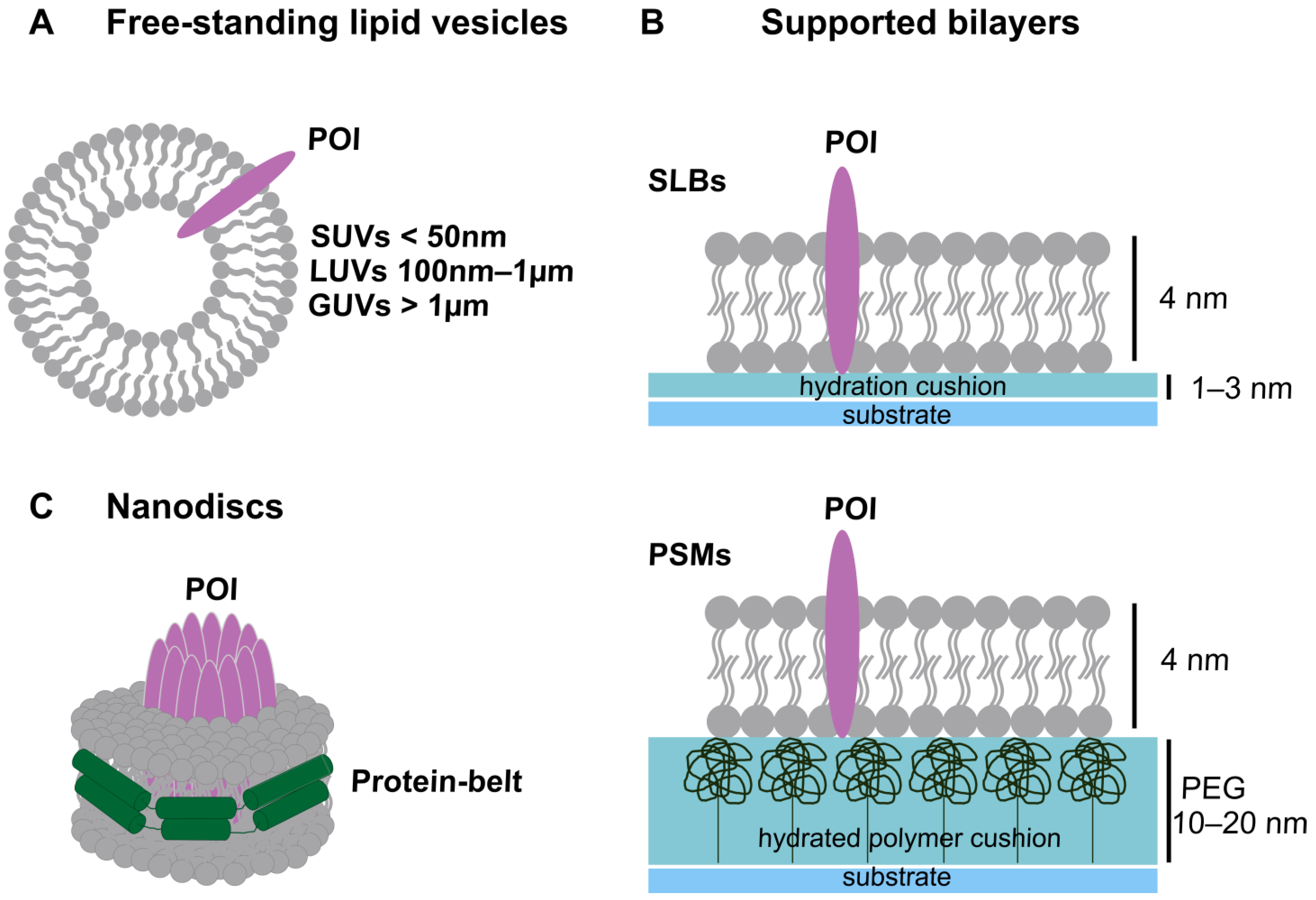
IJMS, Free Full-Text

γ-Hemolysin oligomeric structure and effect of its formation on supported lipid bilayers: An AFM Investigation - ScienceDirect

Assemblies of pore-forming toxins visualized by atomic force microscopy - ScienceDirect

Cryo-EM-based structural insights into supramolecular assemblies of γ- hemolysin from S. aureus reveal the pore formation mechanism - ScienceDirect
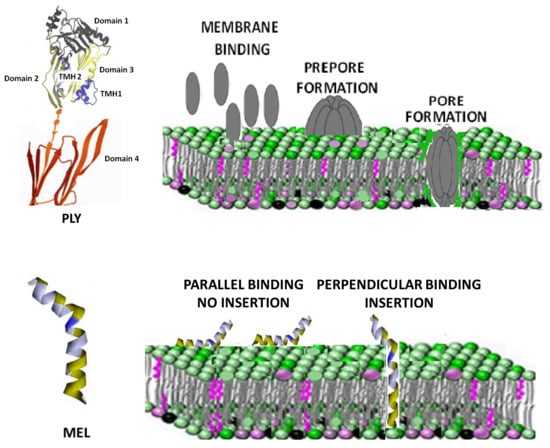
Toxins, Free Full-Text

PDF) Staphylococcus aureus Hemolysins, bi-component Leukocidins, and Cytolytic Peptides: A Redundant Arsenal of Membrane-Damaging Virulence Factors?

Assembling the puzzle: Oligomerization of α-pore forming proteins in membranes - ScienceDirect

Molecular Architecture and Functional Analysis of NetB, a Pore-forming Toxin from Clostridium perfringens - ScienceDirect

α-Hemolysin pore formation into a supported phospholipid bilayer using cell-free expression - ScienceDirect

α-Hemolysin pore formation into a supported phospholipid bilayer using cell-free expression - ScienceDirect
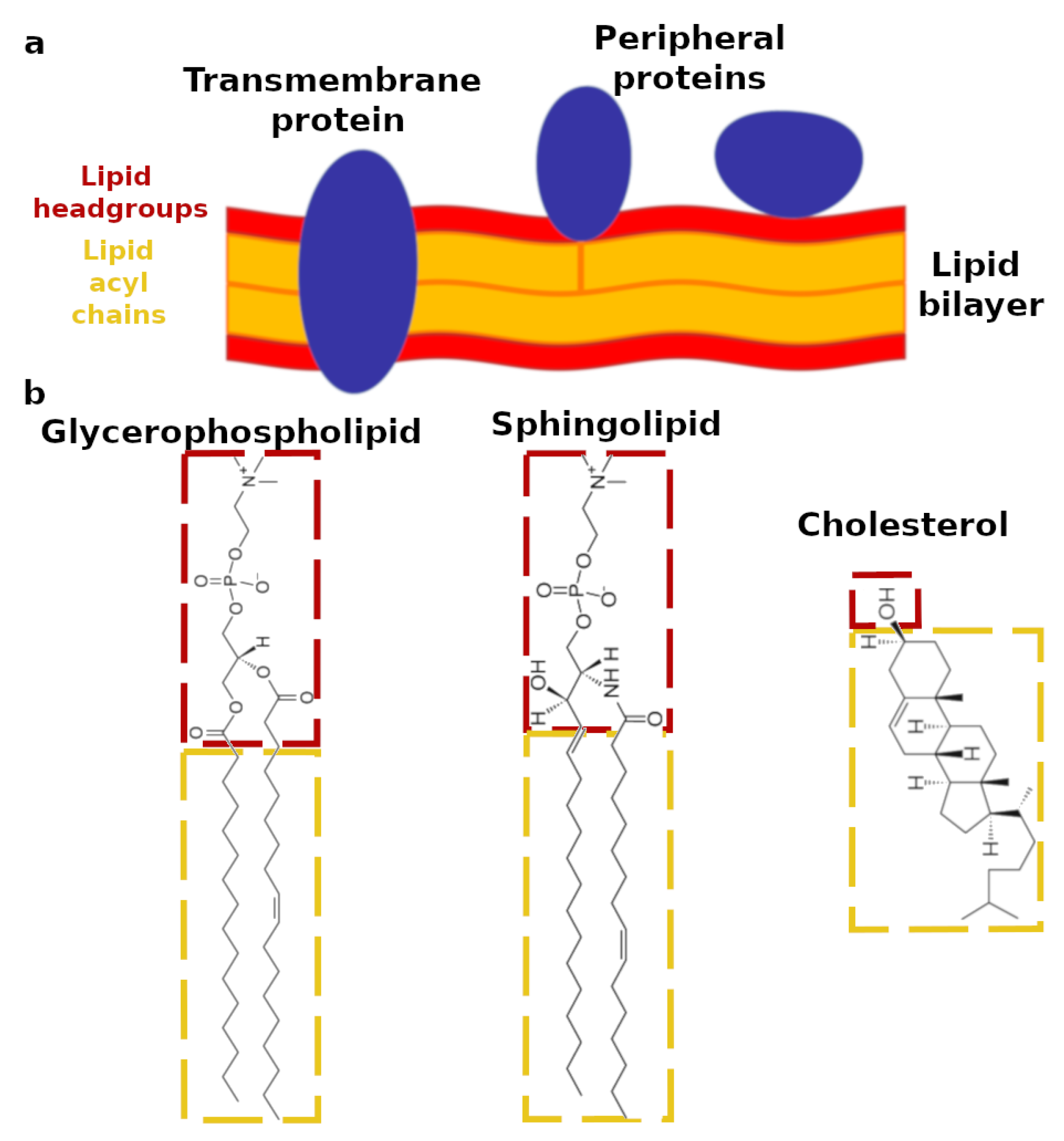
Biomimetics, Free Full-Text
für dich empfohlen
 Analyses of Distances from the Plasma Membrane to Intracellular Actin14 Jul 2023
Analyses of Distances from the Plasma Membrane to Intracellular Actin14 Jul 2023 Membrane and Membrane Processes Learning Activities (Distance Learning)14 Jul 2023
Membrane and Membrane Processes Learning Activities (Distance Learning)14 Jul 2023 Hohe Effizienz Handy Staub Film Entfernen Membran Klebeband LCD Screen Schutz Film Glas Schutz Band - AliExpress14 Jul 2023
Hohe Effizienz Handy Staub Film Entfernen Membran Klebeband LCD Screen Schutz Film Glas Schutz Band - AliExpress14 Jul 2023 In Situ Labeling and Distance Measurements of Membrane Proteins in E. coli Using Finland and OX063 Trityl Labels - Ketter - 2021 - Chemistry – A European Journal - Wiley Online Library14 Jul 2023
In Situ Labeling and Distance Measurements of Membrane Proteins in E. coli Using Finland and OX063 Trityl Labels - Ketter - 2021 - Chemistry – A European Journal - Wiley Online Library14 Jul 2023- Entfernung von organischen Spurenstoffen und Untersuchung von14 Jul 2023
 China Customized Ammoniak Removal Membrane Schütz Modell ETN16X2814 Jul 2023
China Customized Ammoniak Removal Membrane Schütz Modell ETN16X2814 Jul 2023 Die Umkehrosmose (RO) Ist Eine Wasserreinigungstechnologie, Bei14 Jul 2023
Die Umkehrosmose (RO) Ist Eine Wasserreinigungstechnologie, Bei14 Jul 2023 Membrane binding of pore-forming γ-hemolysin components studied at14 Jul 2023
Membrane binding of pore-forming γ-hemolysin components studied at14 Jul 2023 Kühlung von Materie aus Distanz14 Jul 2023
Kühlung von Materie aus Distanz14 Jul 2023 Genetic, cellular, and structural characterization of the membrane14 Jul 2023
Genetic, cellular, and structural characterization of the membrane14 Jul 2023
Sie können auch mögen
 Ladekantenschutz Lackschutzfolie selbstklebend Transparent Folie Limousine L00714 Jul 2023
Ladekantenschutz Lackschutzfolie selbstklebend Transparent Folie Limousine L00714 Jul 2023 Innendämmung: Diese verschiedenen Systeme gibt es14 Jul 2023
Innendämmung: Diese verschiedenen Systeme gibt es14 Jul 2023 Wera 020081 Joker Combination Wrench with Switch - 11/1614 Jul 2023
Wera 020081 Joker Combination Wrench with Switch - 11/1614 Jul 2023 Kühe mit Hörnern: Hornkuh Volksabstimmung in der Schweiz - manager14 Jul 2023
Kühe mit Hörnern: Hornkuh Volksabstimmung in der Schweiz - manager14 Jul 2023 Nachrüstset Ladebooster Votronic 1212-50 universal 5m » NEOVARIA-Shop14 Jul 2023
Nachrüstset Ladebooster Votronic 1212-50 universal 5m » NEOVARIA-Shop14 Jul 2023 VEVOR Dachzelt Truck Lkw Zelt 200 x 165 x 170 cm14 Jul 2023
VEVOR Dachzelt Truck Lkw Zelt 200 x 165 x 170 cm14 Jul 2023 Equipo Multimedia para Audi A4 B8 A5 2009-2016 (8 Core 4 + 64GB14 Jul 2023
Equipo Multimedia para Audi A4 B8 A5 2009-2016 (8 Core 4 + 64GB14 Jul 2023 Auto Styling Auto Lenkrad Schaltwippen Schaltwippen Erweiterung mit Klimaanlage Ring Knauf Abdeckungen für Dodge Challenger 2015 +14 Jul 2023
Auto Styling Auto Lenkrad Schaltwippen Schaltwippen Erweiterung mit Klimaanlage Ring Knauf Abdeckungen für Dodge Challenger 2015 +14 Jul 2023 855762 Brodit ProClip14 Jul 2023
855762 Brodit ProClip14 Jul 2023 Seat Ibiza Ibiza 1.0 Tgi /service Neu /tüv Neu / Garantie Bleu d'occasion, moteur GNV-CNG et boite Manuelle, 62.000 Km - 11.990 €14 Jul 2023
Seat Ibiza Ibiza 1.0 Tgi /service Neu /tüv Neu / Garantie Bleu d'occasion, moteur GNV-CNG et boite Manuelle, 62.000 Km - 11.990 €14 Jul 2023