FDA Allows PM to Market IQOS as 'Reduced Risk' - Tobacco Asia
Von einem Mystery-Man-Autor
Last updated 19 Juni 2024
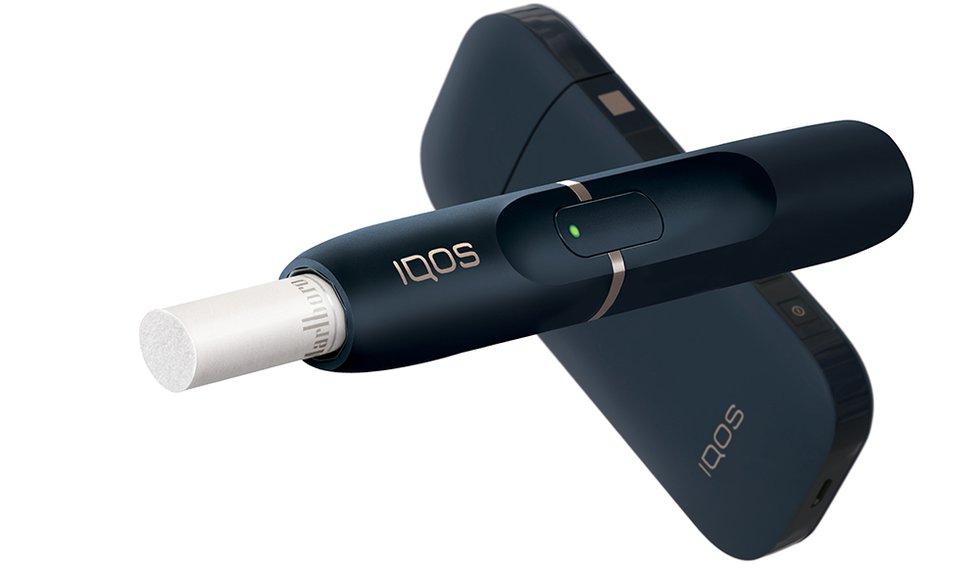
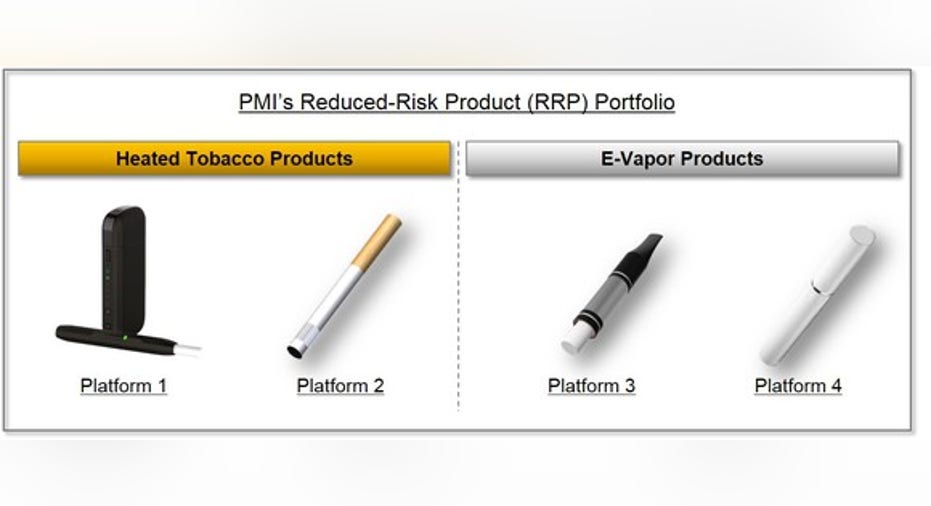
What Philip Morris International's FDA Application Means for iQOS

Heated Tobacco Products: Philip Morris International - TobaccoTactics

IQOS in the U.S.

IQOS Cigarette Alternative Suffers a Blow at FDA Panel - WSJ

Iqos - Wikipedia

FDA Says IQOS Tobacco Products Reduce Exposure to Harmful Chemicals Found In Cigarette Smoke - Reason Foundation

FDA Says PM USA Can Market IQOS as Modified Risk Tobacco Products
Philip Morris's Cigarette Alternative Could Hit U.S. in 2017 - Bloomberg

Tobacco Truth: FDA Approves PMI's IQOS Heat-Not-Burn Tobacco For U.S. Sales

A relentless work for the FDA with PMI's iQOS MRTP application - Vaping Post
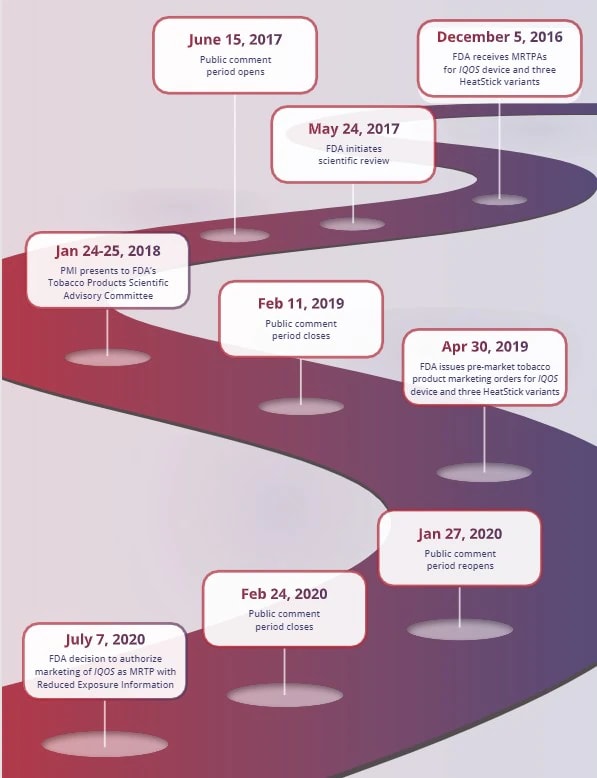
Historic decision made as IQOS Tobacco Heating System is authorized under U.S. MRTP pathway
für dich empfohlen
 HEETS Tabaksticks Selection Mix IQOS14 Jul 2023
HEETS Tabaksticks Selection Mix IQOS14 Jul 2023 Heets von IQOS kaufen: alle Sorten direkt verfügbar14 Jul 2023
Heets von IQOS kaufen: alle Sorten direkt verfügbar14 Jul 2023 HEETS Bronze Selection E-Zigaretten Sticks für IQOS 1 x 20 Stück14 Jul 2023
HEETS Bronze Selection E-Zigaretten Sticks für IQOS 1 x 20 Stück14 Jul 2023 Philip Morris - HEETS für IQOS 3 DUO Kit - Tabaksticks - Tabak14 Jul 2023
Philip Morris - HEETS für IQOS 3 DUO Kit - Tabaksticks - Tabak14 Jul 2023 IQOS ILUMA Azure Blue online kaufen14 Jul 2023
IQOS ILUMA Azure Blue online kaufen14 Jul 2023 Iqos Banque de photographies et d'images à haute résolution - Alamy14 Jul 2023
Iqos Banque de photographies et d'images à haute résolution - Alamy14 Jul 2023 Ook verhitte tabak bevat verslavende nicotine en schadelijke14 Jul 2023
Ook verhitte tabak bevat verslavende nicotine en schadelijke14 Jul 2023 IQOS 3 DUO Kit – Tabakerhitzer – Warm White (in 4 Farben14 Jul 2023
IQOS 3 DUO Kit – Tabakerhitzer – Warm White (in 4 Farben14 Jul 2023 IQOS™ HEETS Bronze Selection Tobacco Sticks14 Jul 2023
IQOS™ HEETS Bronze Selection Tobacco Sticks14 Jul 2023- What do HEETS contain? Is it real tobacco?14 Jul 2023
Sie können auch mögen
 MOTOPOWER MP0515A 12V Car Battery Tester Automotive 100-2000 CCA14 Jul 2023
MOTOPOWER MP0515A 12V Car Battery Tester Automotive 100-2000 CCA14 Jul 2023 TACCTS Auto Mittelarmlehne Pad für BMW Serie 6 Series 6er/M6 F1214 Jul 2023
TACCTS Auto Mittelarmlehne Pad für BMW Serie 6 Series 6er/M6 F1214 Jul 2023 SMART Forfour I (454) Ersatzteilkatalog │ EU-AUTOTEILE Store14 Jul 2023
SMART Forfour I (454) Ersatzteilkatalog │ EU-AUTOTEILE Store14 Jul 2023 Schwangere Auto Sicherheits gurt Einsteller schwangere Frau fahren14 Jul 2023
Schwangere Auto Sicherheits gurt Einsteller schwangere Frau fahren14 Jul 2023 Projecteur principal droit (Côté passager) Mercedes Viano/Vito W639 03-10 - EuropeTuning14 Jul 2023
Projecteur principal droit (Côté passager) Mercedes Viano/Vito W639 03-10 - EuropeTuning14 Jul 2023- Seat Leon - Carbon Air Intake Kit (Ansaugung HG Motorsport14 Jul 2023
 ▷ Kipper Isuzu NPR Autom. 5.2 Ltr. Abrollkipper mit Funkfernbed14 Jul 2023
▷ Kipper Isuzu NPR Autom. 5.2 Ltr. Abrollkipper mit Funkfernbed14 Jul 2023 24V Auto Heizlüfter Abtauen Heizung Auto Elektrische Windschutzscheibe Demister Outdoor Reise LKW Auto Hitze14 Jul 2023
24V Auto Heizlüfter Abtauen Heizung Auto Elektrische Windschutzscheibe Demister Outdoor Reise LKW Auto Hitze14 Jul 2023 COUVERCLE DE RÉSERVOIR essence pour STIHL FS81 FS86 FS88 FS120 FS300 FS350 FS450 EUR 4,80 - PicClick FR14 Jul 2023
COUVERCLE DE RÉSERVOIR essence pour STIHL FS81 FS86 FS88 FS120 FS300 FS350 FS450 EUR 4,80 - PicClick FR14 Jul 2023 Auto Sicherheitsgurt Verlängerung Schnalle Clips14 Jul 2023
Auto Sicherheitsgurt Verlängerung Schnalle Clips14 Jul 2023
