FDA Outlines Generic Safety and Efficacy - Policy and Medicine
Von einem Mystery-Man-Autor
Last updated 05 Juli 2024
FDA Medical Device Classification: Classes and Examples
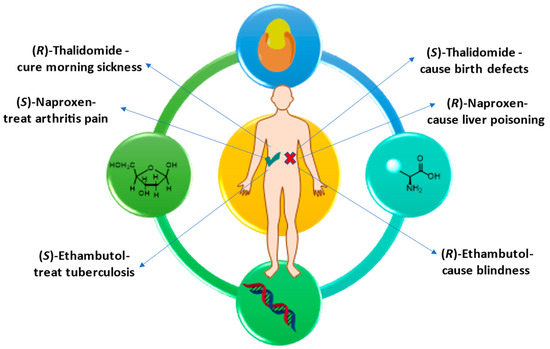
Pharmaceuticals, Free Full-Text

6 Takeaways from the FDA's Complex Generic Drug Product Development Workshop

Drugs, Devices, and the FDA: Part 1: An Overview of Approval Processes for Drugs - ScienceDirect

Foods, Free Full-Text

Machine learning-based quantitative prediction of drug exposure in drug-drug interactions using drug label information

U.S. FDA Approved Drugs from 2015–June 2020: A Perspective

Quality by Design for ANDAs: An Example for Immediate-Release Dosage Forms

FDA's Regulatory Framework for 3D Printing of Medical Devices at the Point of Care Needs More Clarity

A smart hospital-driven approach to precision pharmacovigilance: Trends in Pharmacological Sciences

Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
für dich empfohlen
 What You Need to Know About Generic Drugs14 Jul 2023
What You Need to Know About Generic Drugs14 Jul 2023 5 Ways to Avoid Creating a Generic Resume - Resume Assassin14 Jul 2023
5 Ways to Avoid Creating a Generic Resume - Resume Assassin14 Jul 2023 Cheap Generic Drugs May Not Be Safe for US Troops - Bloomberg14 Jul 2023
Cheap Generic Drugs May Not Be Safe for US Troops - Bloomberg14 Jul 2023 Generic Medicine PCD Franchise Company in India14 Jul 2023
Generic Medicine PCD Franchise Company in India14 Jul 2023 Cipla Generic Pharmaceutical Medicine, For Hospital, Packaging Size: 10 Tbalets at Rs 300/piece in Nagpur14 Jul 2023
Cipla Generic Pharmaceutical Medicine, For Hospital, Packaging Size: 10 Tbalets at Rs 300/piece in Nagpur14 Jul 2023 Generic Brand Definition - Difference from Brand Name14 Jul 2023
Generic Brand Definition - Difference from Brand Name14 Jul 2023 The Generic vs. Brand Name Debate: How Does It Apply to Feed14 Jul 2023
The Generic vs. Brand Name Debate: How Does It Apply to Feed14 Jul 2023 Original Vs Generic Drugs. Is there a difference? Which is better14 Jul 2023
Original Vs Generic Drugs. Is there a difference? Which is better14 Jul 2023- Malarone only £1.40, Malaria Prevention14 Jul 2023
 Generic Medicines: Are Good Or Bad For You?14 Jul 2023
Generic Medicines: Are Good Or Bad For You?14 Jul 2023
Sie können auch mögen
 LST Verschlusskappe Deckel Wischwasserbehälter 207 208 309 406 80714 Jul 2023
LST Verschlusskappe Deckel Wischwasserbehälter 207 208 309 406 80714 Jul 2023 Mollers Cod Liver Oil - Healthspan14 Jul 2023
Mollers Cod Liver Oil - Healthspan14 Jul 2023 Autolack-Tester PRO - Autolacktester - magnetischer Lacktester14 Jul 2023
Autolack-Tester PRO - Autolacktester - magnetischer Lacktester14 Jul 2023 Automatischer Batterietrenner CSB 96-SP mit Netzteil von „+key“ 40296514 Jul 2023
Automatischer Batterietrenner CSB 96-SP mit Netzteil von „+key“ 40296514 Jul 2023 Original Kotflügel vorne links gebraucht VW TIGUAN II + ALLSPACE14 Jul 2023
Original Kotflügel vorne links gebraucht VW TIGUAN II + ALLSPACE14 Jul 2023 1pc 12V/24V Auto Zigarettenanzünder Stecker Auf Buchse 120W/10A Schwerlast-Verlängerungskabel Kompatibel Mit Luftkompressor Kühlschrank Auto-Staubsauger - Temu Austria14 Jul 2023
1pc 12V/24V Auto Zigarettenanzünder Stecker Auf Buchse 120W/10A Schwerlast-Verlängerungskabel Kompatibel Mit Luftkompressor Kühlschrank Auto-Staubsauger - Temu Austria14 Jul 2023 Schalldämmung Auto kaufen? CROP Paints & NonPaints14 Jul 2023
Schalldämmung Auto kaufen? CROP Paints & NonPaints14 Jul 2023 Sirenenalarm am ersten Mittwoch des Monats: Wozu dient dieses Signal und wie ist es zu interpretieren?14 Jul 2023
Sirenenalarm am ersten Mittwoch des Monats: Wozu dient dieses Signal und wie ist es zu interpretieren?14 Jul 2023- Rotinger Graphite Sport-Bremsscheiben Satz Vorderachse - Suzuki14 Jul 2023
- Auspuffanlage komplett BMW EMW 340 BMW 326-3400210100014 Jul 2023


