Evaluation of eight commercial Zika virus IgM and IgG serology assays for diagnostics and research
Von einem Mystery-Man-Autor
Last updated 01 Juni 2024
Several commercial Zika virus (ZIKV) serology assays have been developed since the recognition of ZIKV outbreaks as a Public Health Emergency of International Concern in 2016. However, test interpretation for ZIKV serology can be challenging due to antibody cross-reactivity with other flaviviruses like dengue virus (DENV). Therefore, we sought to evaluate the performance of eight commercially available ZIKV IgM and IgG assays across three testing platforms, namely, immunochromatographic tests (ICT), ELISAs and immunofluorescence tests (IIFT). The test panel comprised of 278 samples, including acute and convalescent sera or plasma from ZIKV-confirmed, DENV-confirmed, non-ZIKV and non-DENV patients, and residual sera from healthy blood donors. The ZIKV IgM and IgG serology assays yielded higher test sensitivities of 23.5% - 97.1% among ZIKV convalescent samples as compared to 5.6% - 27.8% among ZIKV acute samples; the test specificities were 63.3% - 100% among acute and convalescent DENV, non-DENV samples. Among the ELISAs and IIFTs, the Diapro ZIKV IgM ELISA demonstrated high test sensitivity (96%) and specificity (80%) when tested on early convalescent samples, while the Euroimmun ZIKV IgG ELISA yielded the highest test specificity of 97% - 100% on samples from non-ZIKV patients and healthy blood donors. For rapid ICTs, the LumiQuick IgM rapid ICT yielded low test sensitivity, suggesting its limited utility. We showed that commercial ZIKV IgM and IgG serology assays have differing test performances, with some having moderate to high test sensitivities and specificities when used in a dengue endemic setting, although there were limitations in IgG serology.

Zika virus: A primer for clinicians

Zika Virus Workup: Approach Considerations, Laboratory Studies

Diagnostics for Zika virus on the horizon

Zika virus

Human Anti-Dengue virus IgG ELISA Kit (ab108728)

Natural infection by Zika virus but not DNA vaccination

Rapid Detection and One-Step Differentiation of Cross-Reactivity

Seroprevalence of Zika virus (ZIKV) antibodies in Central Vietnam
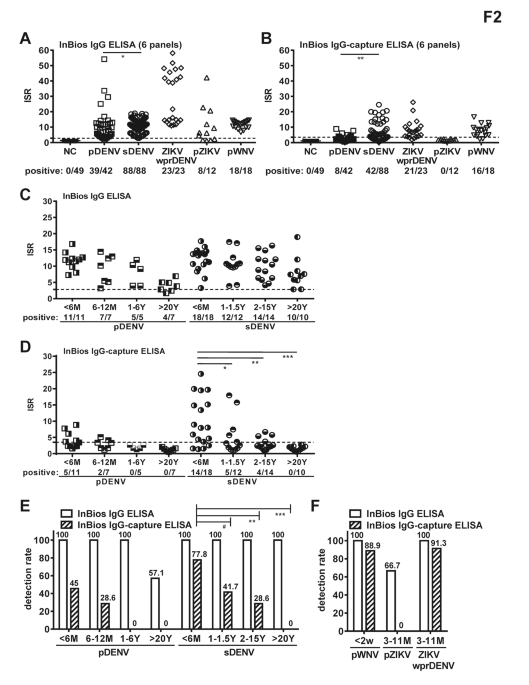
Comparing the performance of dengue virus IgG and IgG-capture
für dich empfohlen
 Test für medizinische Studiengänge I: Originalversion I des TMS - Consulting, Itb - Livres14 Jul 2023
Test für medizinische Studiengänge I: Originalversion I des TMS - Consulting, Itb - Livres14 Jul 2023 envihab - Medizinische Tests für Astronauten / If you wan…14 Jul 2023
envihab - Medizinische Tests für Astronauten / If you wan…14 Jul 2023 Wie wird ein Corona-Test durchgeführt?14 Jul 2023
Wie wird ein Corona-Test durchgeführt?14 Jul 2023 Test für medizinische Studiengänge (TMS) – Informationen und Anmeldung zum Mediziner-Test14 Jul 2023
Test für medizinische Studiengänge (TMS) – Informationen und Anmeldung zum Mediziner-Test14 Jul 2023 Saraschandra Vallabhajosyula, MD MSc on X: Came across this interesting technique of guidewire fragment retrieval by @swissCTO, @AlexAchimMD, @LKrivoshei published in @ESC_Journals Case Reports - Thoughts from CHIP/CTO14 Jul 2023
Saraschandra Vallabhajosyula, MD MSc on X: Came across this interesting technique of guidewire fragment retrieval by @swissCTO, @AlexAchimMD, @LKrivoshei published in @ESC_Journals Case Reports - Thoughts from CHIP/CTO14 Jul 2023 MedlinePlus: Medical Tests14 Jul 2023
MedlinePlus: Medical Tests14 Jul 2023 Medical Tests & Procedures - Harvard Health14 Jul 2023
Medical Tests & Procedures - Harvard Health14 Jul 2023 5 important blood tests beyond the basics - Harvard Health14 Jul 2023
5 important blood tests beyond the basics - Harvard Health14 Jul 2023:max_bytes(150000):strip_icc()/coronavirus-test-AdobeStock_228118399-50ecfdd0666543bab13cfe7438c96144.jpg) Types of COVID Tests: What You Need to Know14 Jul 2023
Types of COVID Tests: What You Need to Know14 Jul 2023 What Are Pre-Medical Tests in Health Insurance?14 Jul 2023
What Are Pre-Medical Tests in Health Insurance?14 Jul 2023
Sie können auch mögen
 Leder PU auto sitz abdeckung 4 Saison Für nissan note juke qashqai j10 almera n16 x-trail t31 navara d40 stil 5 farben - AliExpress14 Jul 2023
Leder PU auto sitz abdeckung 4 Saison Für nissan note juke qashqai j10 almera n16 x-trail t31 navara d40 stil 5 farben - AliExpress14 Jul 2023 sourcing map Durite Carburant Tuyau 6mm(15/64) ID x 9mm(23/64) Diamètre Exterieur 8.2ft/2.5M Essence Caoutchouc nitrile Eau Tube Conduite Moteur : : Auto et Moto14 Jul 2023
sourcing map Durite Carburant Tuyau 6mm(15/64) ID x 9mm(23/64) Diamètre Exterieur 8.2ft/2.5M Essence Caoutchouc nitrile Eau Tube Conduite Moteur : : Auto et Moto14 Jul 2023- Joie i-Snug Babyschale in laurel grün (MAXI COSI)14 Jul 2023
 Multifunktionale Batteriebox Outdoor Portable Batteriebox Rv - Temu Austria14 Jul 2023
Multifunktionale Batteriebox Outdoor Portable Batteriebox Rv - Temu Austria14 Jul 2023- Schließzylinder für VW T5 kaufen ▷ AUTODOC Online-Shop14 Jul 2023
 Braised pork 2PcsHoodie Gear Shift Cover, Funny Gear Shift Knob Hoodie, Mini Hoodie for Car Shifter, Automotive Interior Accessories, Shift Knobs Fashionable Hooded Shirt Car : : Auto & Motorrad14 Jul 2023
Braised pork 2PcsHoodie Gear Shift Cover, Funny Gear Shift Knob Hoodie, Mini Hoodie for Car Shifter, Automotive Interior Accessories, Shift Knobs Fashionable Hooded Shirt Car : : Auto & Motorrad14 Jul 2023 Gasdruckfeder mit 200N / 20 kg Ausschubkraft und 536mm Länge, Gasdruckdämpfer, Gasfeder : : Auto & Motorrad14 Jul 2023
Gasdruckfeder mit 200N / 20 kg Ausschubkraft und 536mm Länge, Gasdruckdämpfer, Gasfeder : : Auto & Motorrad14 Jul 2023 Leim- und Filmentferner 90mm Vanille-Scheibe14 Jul 2023
Leim- und Filmentferner 90mm Vanille-Scheibe14 Jul 2023 Hazet Werkzeug-Sortiment 148-teilig - 1109636014 Jul 2023
Hazet Werkzeug-Sortiment 148-teilig - 1109636014 Jul 2023 Karneval der Stars 53“ von Karneval der Stars – Apple Music14 Jul 2023
Karneval der Stars 53“ von Karneval der Stars – Apple Music14 Jul 2023

