Circulating mitochondria promoted endothelial cGAS-derived neuroinflammation in subfornical organ to aggravate sympathetic overdrive in heart failure mice, Journal of Neuroinflammation
Von einem Mystery-Man-Autor
Last updated 19 Juni 2024
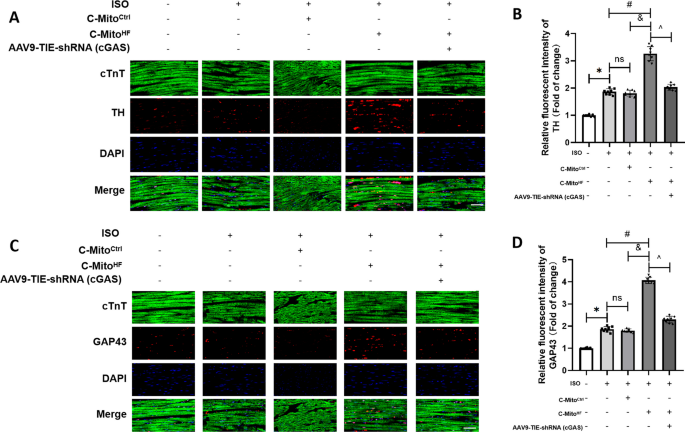

Figure S2. Experimental microglia depletion by PLX3397 (intragastric

Tumor cells killed by ultrasound-responsive chemotherapeutics triggered

Frontiers Microglia-mediated inflammatory destruction of neuro- cardiovascular dysfunction after stroke

ALDH2 contributes to melatonin-induced protection against APP/PS1 mutation-prompted cardiac anomalies through cGAS-STING-TBK1-mediated regulation of mitophagy

Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration

Renin-angiotensin system influence on blood pressure. ACE; Angiotensin
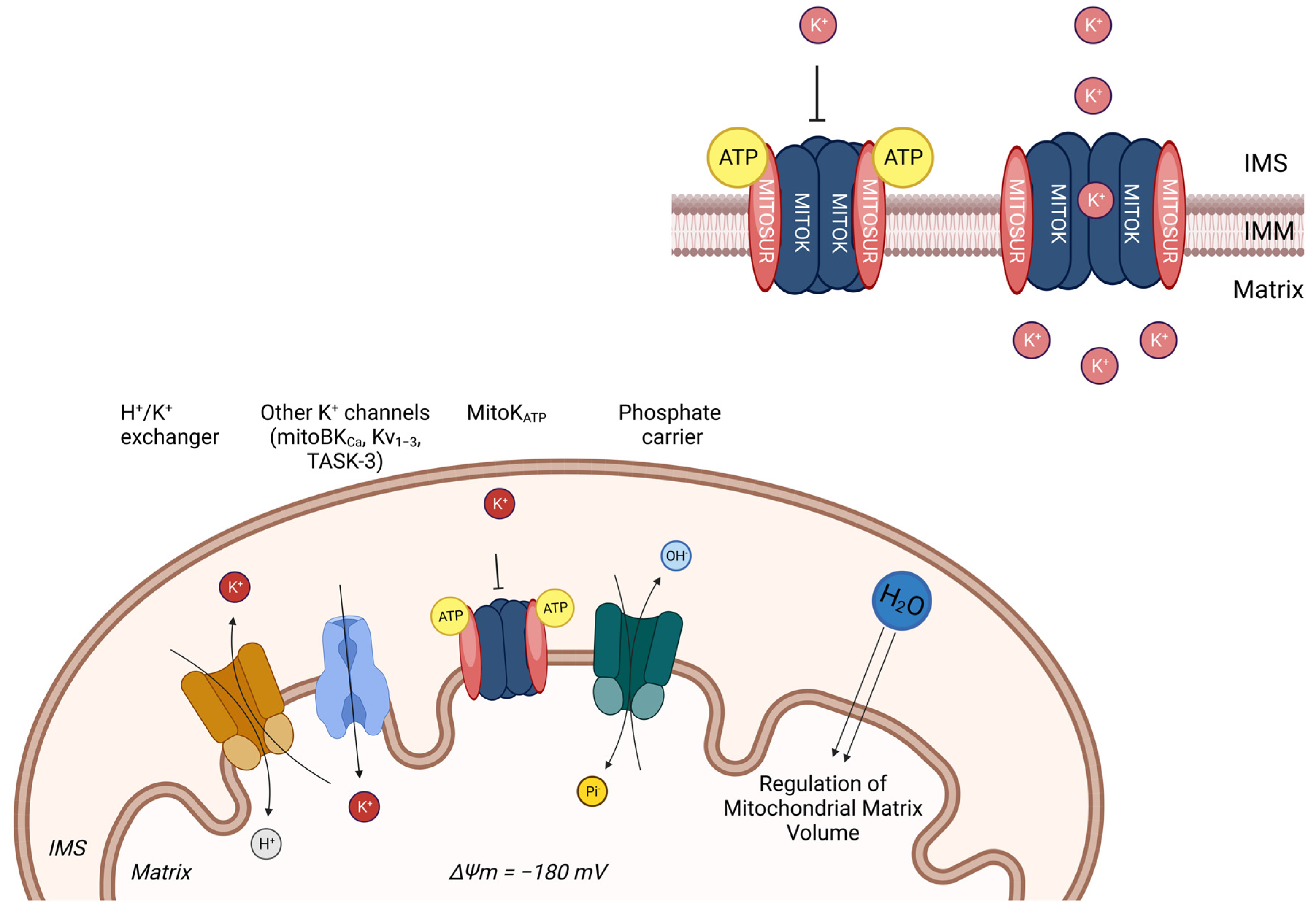
IJMS, Free Full-Text

The experimental flow chart of the study.

LCSAA attenuated AMI‐induced astrocyte and neuronal activation in the

Circulating mitochondria promoted endothelial cGAS-derived neuroinflammation in subfornical organ to aggravate sympathetic overdrive in heart failure mice, Journal of Neuroinflammation

Circulating mitochondria promoted endothelial cGAS-derived neuroinflammation in subfornical organ to aggravate sympathetic overdrive in heart failure mice, Journal of Neuroinflammation

Macrophages induce cardiomyocyte ferroptosis via mitochondrial transfer - ScienceDirect
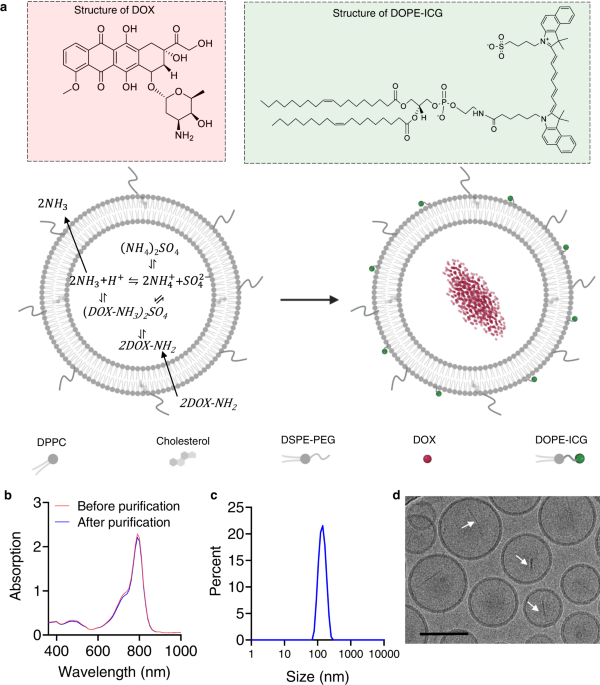
Ultrasound-responsive low-dose doxorubicin liposomes trigger mitochondrial DNA release and activate cGAS-STING-mediated antitumour immunity
für dich empfohlen
 9 x AAB SIGNAL GAS HORN 750ml - GAS-HUPE, LUFT-HUPE, DRUCKLUFT-HUPE, HORN-S14 Jul 2023
9 x AAB SIGNAL GAS HORN 750ml - GAS-HUPE, LUFT-HUPE, DRUCKLUFT-HUPE, HORN-S14 Jul 2023 Radon: Truth vs. myth14 Jul 2023
Radon: Truth vs. myth14 Jul 2023 Alarm horn for storage and disposal tank TA 700/100014 Jul 2023
Alarm horn for storage and disposal tank TA 700/100014 Jul 2023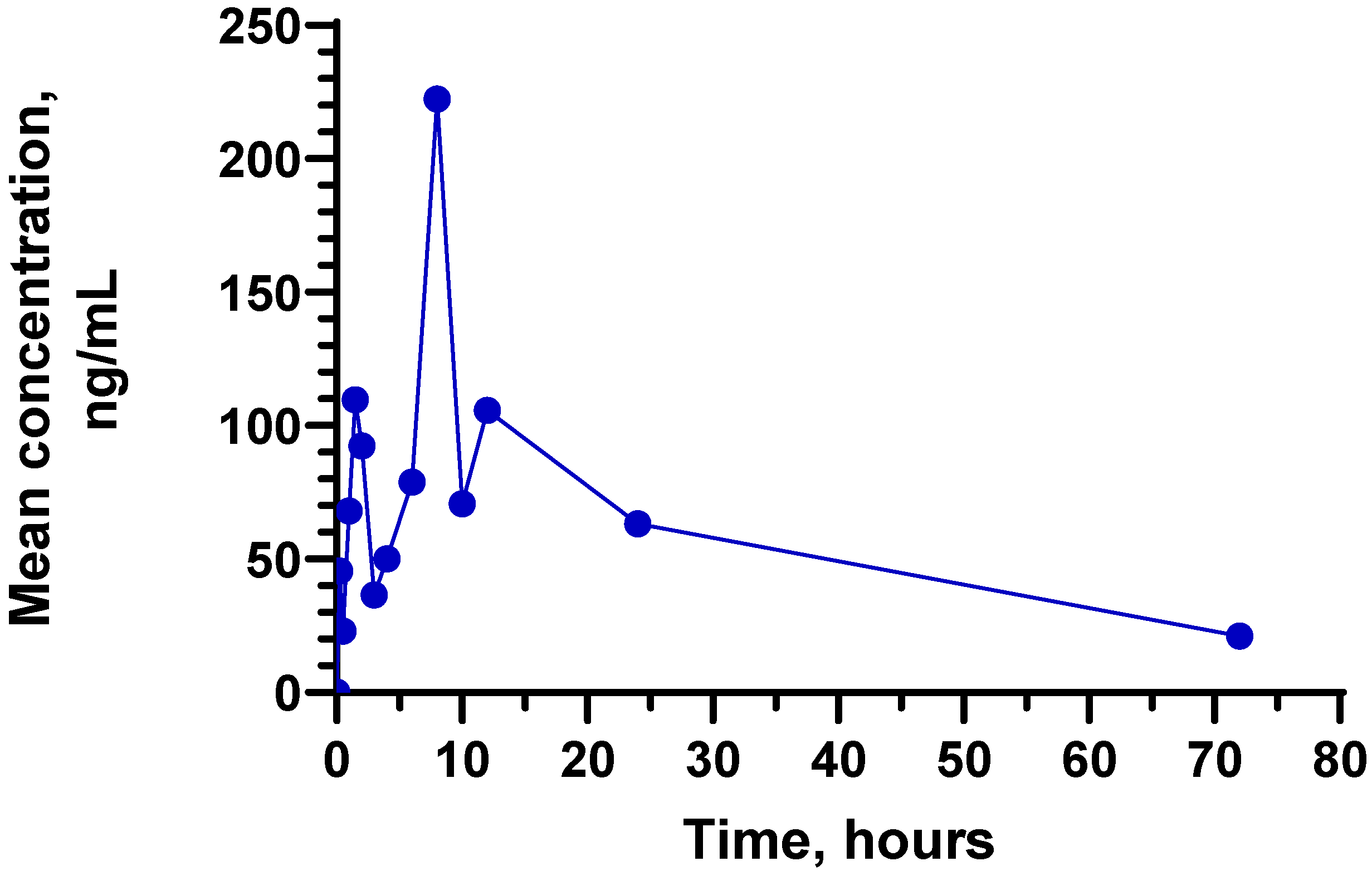 Molecules, Free Full-Text14 Jul 2023
Molecules, Free Full-Text14 Jul 2023 Image 282 of Dun and Bradstreet Reference Book: January, 1921; Vol14 Jul 2023
Image 282 of Dun and Bradstreet Reference Book: January, 1921; Vol14 Jul 2023 ADGEO - Radon metrology for use in climate change observation and14 Jul 2023
ADGEO - Radon metrology for use in climate change observation and14 Jul 2023 DoubleHit GF-905 Ersatzflasche, Rundumleuchten, Sondersignalanlagen, Blaulicht, Gelblicht, Blitzer, Sirene, LED, Autozubehör online bestellen und kaufen14 Jul 2023
DoubleHit GF-905 Ersatzflasche, Rundumleuchten, Sondersignalanlagen, Blaulicht, Gelblicht, Blitzer, Sirene, LED, Autozubehör online bestellen und kaufen14 Jul 2023 BAAS Zigarettenanzünder-Dose mit Federdeckel ZA22 Passend für (Details) Zigarettenanzünder14 Jul 2023
BAAS Zigarettenanzünder-Dose mit Federdeckel ZA22 Passend für (Details) Zigarettenanzünder14 Jul 2023 Thermoluminescence measurements of eye-lens dose in a multi-centre stereotactic radiosurgery audit - ScienceDirect14 Jul 2023
Thermoluminescence measurements of eye-lens dose in a multi-centre stereotactic radiosurgery audit - ScienceDirect14 Jul 2023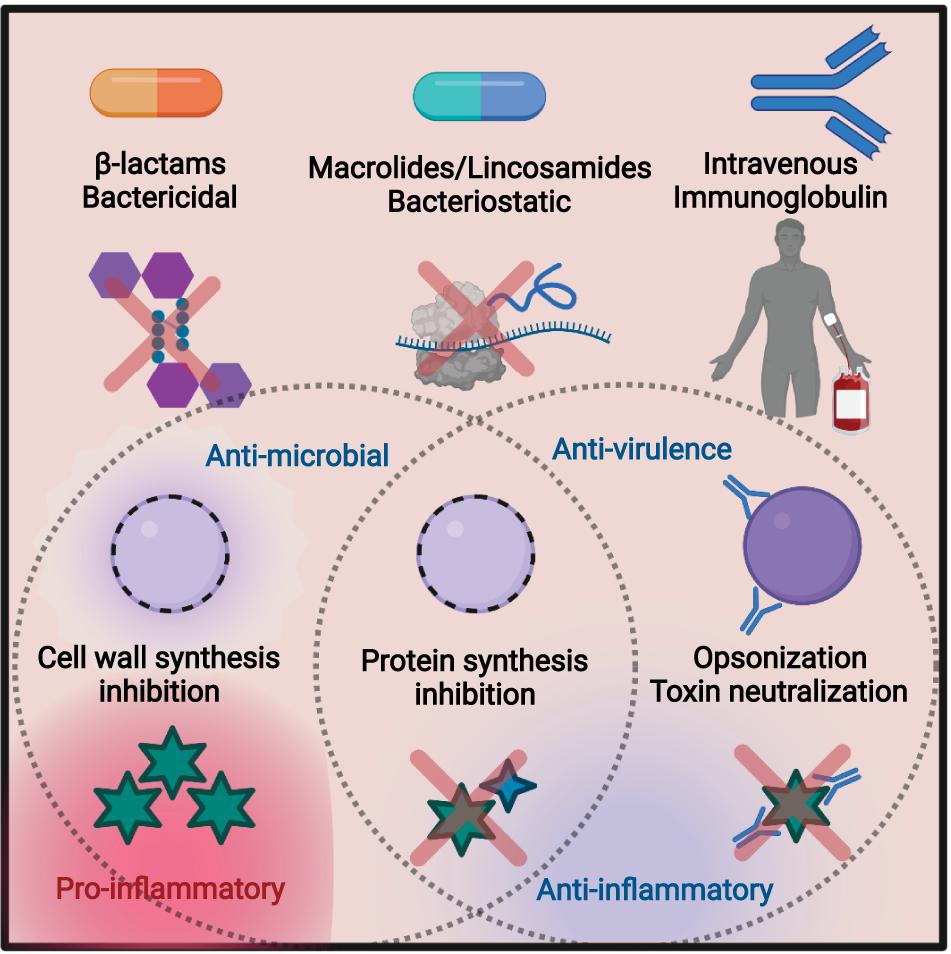 Frontiers Antibiotic Treatment, Mechanisms for Failure, and Adjunctive Therapies for Infections by Group A Streptococcus14 Jul 2023
Frontiers Antibiotic Treatment, Mechanisms for Failure, and Adjunctive Therapies for Infections by Group A Streptococcus14 Jul 2023
Sie können auch mögen
 Rgb-umgebungslampe, Auto-led-neon-kaltlicht, Auto-innenraum14 Jul 2023
Rgb-umgebungslampe, Auto-led-neon-kaltlicht, Auto-innenraum14 Jul 2023- Drucksensor Klimaanlage für AUDI A4 B6 Avant (8E5) 1.9 TDI 2001-2004 Diesel 130PS AVF14 Jul 2023
 Auto-Rückspiegel-Regenfolie, Blendschutz-Antibeschlagfolie für Rückspiegel14 Jul 2023
Auto-Rückspiegel-Regenfolie, Blendschutz-Antibeschlagfolie für Rückspiegel14 Jul 2023 Anhängerkupplung Ignis – Autohaus Otto Griesbeck GmbH14 Jul 2023
Anhängerkupplung Ignis – Autohaus Otto Griesbeck GmbH14 Jul 2023 Abzieher-Set mit Ständer - 5-teilig - bis 7,5 t Zugkraft - bis 200 mm Spanntiefe14 Jul 2023
Abzieher-Set mit Ständer - 5-teilig - bis 7,5 t Zugkraft - bis 200 mm Spanntiefe14 Jul 2023 Premium Faraday Box Autoschlüsseletui Tasche RFID Schloss14 Jul 2023
Premium Faraday Box Autoschlüsseletui Tasche RFID Schloss14 Jul 2023 Kuga MK2 Vordere rechte Tür Dichtung 201214 Jul 2023
Kuga MK2 Vordere rechte Tür Dichtung 201214 Jul 2023- GRANIT Warntafel-Satz, Raiffeisen Agrar14 Jul 2023
 simson schwalbe - Simson Schwalbe - Sticker14 Jul 2023
simson schwalbe - Simson Schwalbe - Sticker14 Jul 2023 Yamaha Roller D'elight YEC-50 Batterieladegerät YME-YEC50-EU-0014 Jul 2023
Yamaha Roller D'elight YEC-50 Batterieladegerät YME-YEC50-EU-0014 Jul 2023
